
One of the most important environmental factors affecting flowering induction is Photoperiod. Photoperiod is defined as the time plants are exposed to light in a daily cycle.
Continue reading Photoperiod and Flowering
One of the most important environmental factors affecting flowering induction is Photoperiod. Photoperiod is defined as the time plants are exposed to light in a daily cycle.
Continue reading Photoperiod and Flowering
When working inside a growing system such as a greenhouse or a plant factory system we must have enough knowledge about light management. When we understand light we can make smart decisions to promote the best environmental conditions to promote growth, development and yield in our crops.
Continue reading How to calculate the light a crop is receiving in the greenhouse
All crops have specific light requirements. By knowing minimum and optimum levels of light for our crop we can manage our environment to improve crop performance. When the ambient light exposure is below the minimum requirements we need to apply supplemental lighting in order to maintain our crop production at a healthy and consistent pace.
Continue reading Mastering Light Management: Optimizing Crop Performance
We all know light is one of the most important factors affecting plant growth and development. But, how can we know if the use of artificial lighting will improve our production and our income? And how can we select the best source of light?
Continue reading When should I invest in artificial lighting? How do I select the best lighting options?
Light is one of the most important factors growing any crop. But, how can you be sure that your plants are receiving enough light? There is a way to measure the light intensity received by your plants.
Continue reading Daily Light Integral: Are your plants receiving the right amount of light?
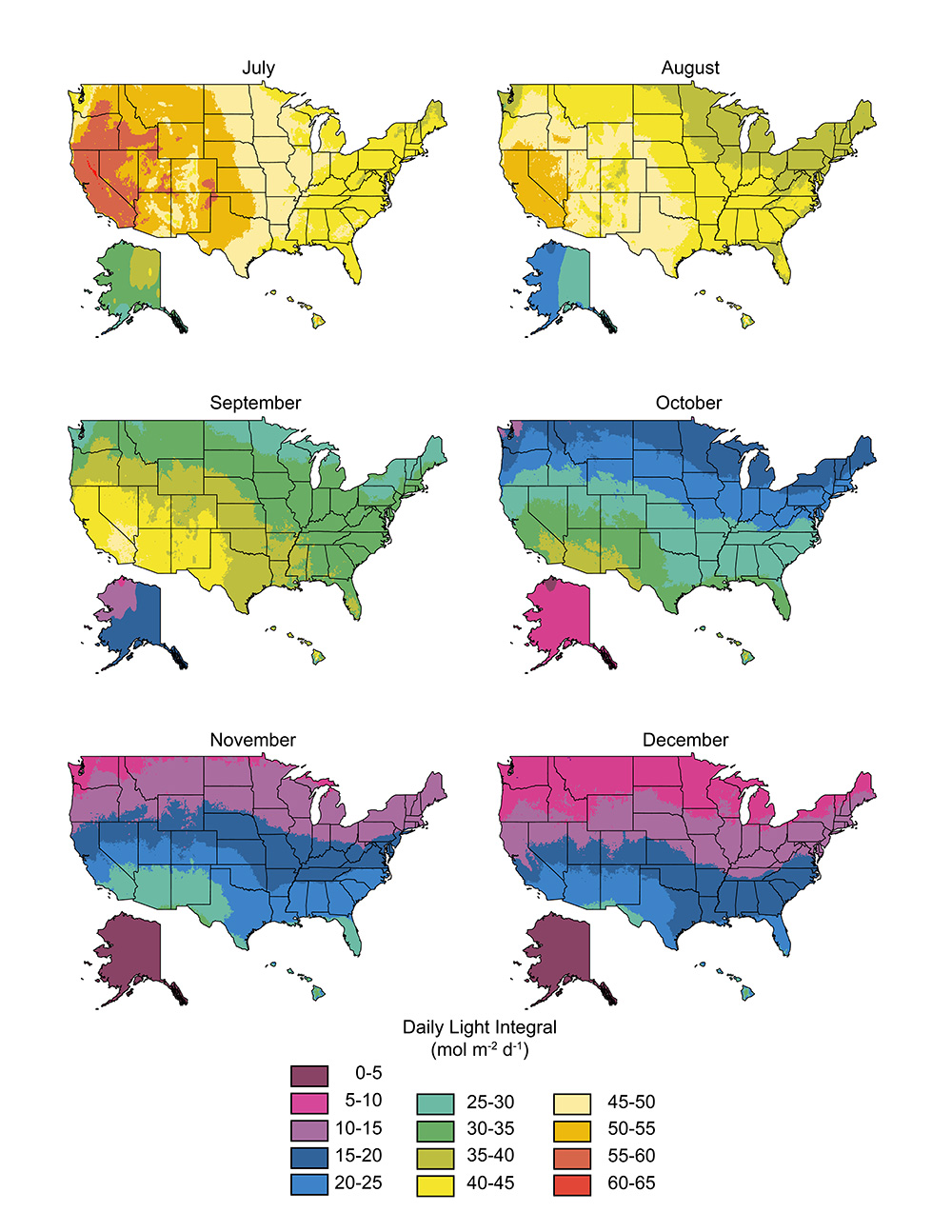
Daily light integral (DLI) is the amount of photosynthetically active radiation (PAR) received each day as a function of light intensity and duration. DLI maps display the ambient light delivered daily during each month across the entire United States. The original maps released in 2002 were researched and developed by Jim Faust at Clemson University and Joanne Logan at the University of Tennessee.
Continue reading Updated daily light integral (DLI) maps now available

“LEDs are now available to deliver all blue, all red, all green, all yellow light or mixtures,” said University of Tennessee plant sciences professor Dean Kopsell. “White LEDs are almost a broad spectrum light source. White LEDs are actually mostly blue light with a little bit of red, yellow and green light with a white phosphor over them.”
Kopsell and his colleagues at the University of Tennessee are studying the impact individual types of light can have on the nutritional qualities of edible crops. Their work is focusing on crops that can be produced relatively quickly in 25-35 days, including microgreens and baby greens. They have also begun looking at some herbal crops including basil, tarragon and chives.
“Some of the unique things we are finding are when we change the light quality environment, going away from broad band light sources like fluorescent, incandescent and HIDs, and exposing plants to narrow band wavelengths of red and blue light, many things are changing in the plants. These narrow bands of light are having an effect on several plant quality parameters from a metabolic standpoint.”
University of Tennessee researchers have found that exposing plants to narrow wavelengths of the light spectrum has resulted in the increased production of antioxidants and anti-carcinogenic compounds within the plants.
“What is even more interesting is some of the primary metabolites like the mineral nutrients are also increasing,” Kopsell said. “We are shifting the light ratios and putting more blue light into the mix. Blue light is close to the ultraviolet (UV) range and has higher energy values than red light. Because of the higher energy level associated with blue light, the more blue light we are exposing the plants to, it seems the more significant the results are on nutritional values.
“We haven’t got hard data yet, but everything that we can see, smell and taste, these blue lights not only affect nutrient uptake, and anti-oxidant metabolism, but they also affect aromatic compounds and flavor compounds. They make them more intense.”
Although researchers have only recently begun to study the impact of narrow light wavelengths on plant physiology, Kopsell said this will be the major use of LEDs in future applications.
“Not only is a grower going to be able to select the type of light and intensity from the LED manufacturer, but eventually the grower will know when is the critical time to apply a specific amount of light to a crop. One of the things that we have seen with these short term crops is using the light as a finishing-off treatment. The crops are grown under regular light conditions like any grower would have the ability to do and then just before harvest the plants would receive a specific type of light for a certain period of time. This light treatment would stimulate the plant physiology uptake and metabolism right before the plants go to the retail market.”
Kopsell said research exposing leafy brassicas to blue light prior to harvest has intensified pigments and green leaf color.
“We increased the green pigments in the leaves so that they looked more vibrant,” he said. “Other research has shown that UV light increases the anthocyanin compounds in leaf lettuce. Providing a little UV light, which is blocked out in most greenhouse environments, at the right time, a grower can get a crop to color up quickly before the plants are shipped out. What we have done with leafy greens to intensify the color of the leaves can also be done with petal tissue. By changing the light quality a grower could get more vibrant flower colors.”
Kopsell said whether plants are grown outdoors, in a greenhouse or in a closed controlled environment with artificial light, the plants are using specific wavelengths from the available light source.
“Horticulture, floriculture and agronomic researchers know how much light is needed in order to produce crops with broad spectrum light,” he said. “The million dollar question that hasn’t been answered is how much light is needed from LEDs to achieve that same level of production? It is going to be less than the daily light integral (DLI) from a broad spectrum light source. But, right now we can’t tell you how much less it’s going to be.
 |
| University of Tennessee studies have shown LED grow lights provide a less stressful light environment for plants. |
“Providing specific types of red and blue light, the amount of stress on plants is reduced because the plants don’t have to tolerate the light not being used for metabolism and physiology,” he said. “We have data that shows LEDs provide a less stressful light environment for plants. So we have to determine how much less light is needed. It is going to require an extra level of management to know what kind of light, how much light and when to apply it. Growers are going to be able to use LEDs to fine tune the light environment. It’s going to depend on the crop, how it’s being grown, where it’s being grown and how the crop will be used. Is it an ornamental, edible or medicinal crop? It’s not going to be as easy as sticking a seed or cutting into a substrate and letting Mother Nature take control. It’s really going to take some fine tune management. But the future looks bright so far.”
For more: Dean Kopsell, University of Tennessee, Plant Sciences Department, Institute of Agriculture, Knoxville, TN 37996-4561; (865) 974-1145; dkopsell@utk.edu.

Greenhouse lettuce can be a successful container or
hydroponic crop for ornamental plant growers looking to give edibles a try.
Mattson said. “Grown as a
container crop, lettuce is relatively similar to petunia. However, lettuce has somewhat
greater calcium needs. Growers can produce a relatively good crop of lettuce in
containers, if they use a complete fertilizer at a moderate strength of 150
parts per million nitrogen.”
Leaf tipburn is a physiological disorder that can occur
when growing greenhouse lettuce. It can greatly impact the salability of a
crop.
 |
| Calcium tipburn in lettuce is not a result of a lack of calcium supplied to the plants, but an inability of the plants to transport enough calcium to the young leaves. |
Mattson has been able to grow a relatively good crop of
container-grown lettuce using granular organic fertilizers incorporated into
the growing medium.
One strategy that Mattson recommends growers do periodically
is to monitor the electrical conductivity (EC) and pH levels.
 |
| Monitoring electrical conductivity (EC) can help avoid under fertilizing lettuce plants, which show yellow lower leaves caused by nitrogen deficiency. |
Mattson said light and temperature are going to be the
drivers for how long it takes to finish a lettuce crop. Whether a grower is
producing the crop in containers with growing medium or hydroponically
shouldn’t have any effect on the length of production.
For more: Neil
Mattson, Cornell University, School of Integrative Plant Science; (607)
255-0621; nsm47@cornell.edu.
Visit our corporate website at https://hortamericas.com

Researchers at Purdue University are finding LEDs can
have positive effects on both ornamentals and leafy vegetables.
By David Kuack
No need for
sunlight
Lopez and former graduate student Wesley Randall found
that greenhouse-grown seedling plugs of impatiens, marigold, petunia, vinca and
zonal geranium did as well or better when supplemented with LEDs compared to
plugs supplemented with light from high pressure sodium lamps. What Lopez found
surprising was the quality of the plugs produced in a growth room with LEDs as
the only light source.
Lopez said many of the annual spring bedding plants grown
in greenhouses in northern climates are produced under low light levels. The
result is that some plants don’t produce the same intense foliage colors that
they would if they were grown outdoors.
Seeing the positive results that occurred with LEDs and
purple fountain grass, Lopez and PhD student W. Garrett Owen expanded the
research to red leaf lettuce to see if they could produce a similar response.
Another study conducted by graduate students Joshua
Gerovac and Joshua Craver looked at the effect of LEDs on the growth of three
different microgreen species (kohlrabi, mustard and mizuna) in an indoor
multilayer production system. The study included three different light
qualities and three different DLIs (light quantity).
Lopez said the ideal vertical LED light module would
contain all of the wavelength colors.
For more:
Roberto Lopez, Purdue University, Department of Horticulture and Landscape
Architecture; (765) 496-3425; rglopez@purdue.edu;
https://ag.purdue.edu/hla/lopezlab/Pages/default.aspx.
Visit our corporate website at https://hortamericas.com
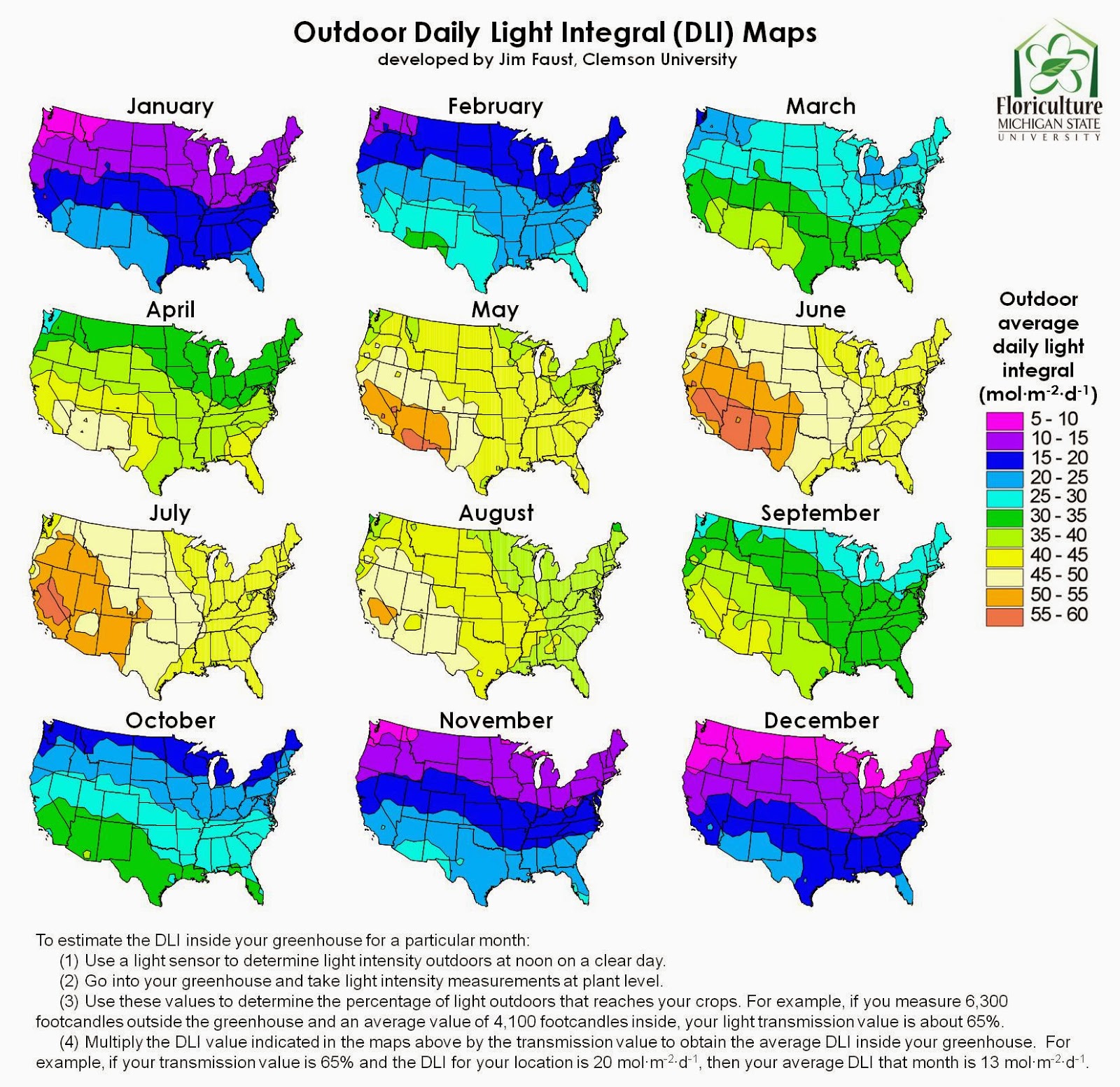
Measuring daily light integral (DLI) provides a more
accurate reading of the amount of photosynthetic light being received by
plants.
Do you measure the light in your greenhouse to ensure
your plants are receiving an adequate amount of light? If you are using a
footcandle meter to measure the light intensity you are not getting a true
measurement of the amount of light received by the plants. Measuring the light
in footcandles or micromoles per square meter per second (µmol/m2/s) is
a measurement of instantaneous light, that is, the amount of light at the time
the measurement is made. An instantaneous measurement made under sunny or cloudy
conditions may not provide an accurate evaluation of the total amount of light
perceived by the plants over the course of a day.
Currey said another issue with measuring light with a
footcandle meter is that it measures the light that is visible to the human
eye.
Currey said it is the total amount of photosynthetic
light that is going to impact how a greenhouse crop is going to grow.
Researchers at Clemson University used light measurements
collected by the National Oceanic Atmospheric Administration to developed
monthly DLI maps for the United States.
Currey said some growers are using DLI, their
environmental controls and supplemental lighting to provide their plants with optimum
PAR levels.
For more: Christopher
Currey, Iowa State University, Department of Horticulture; (515) 294-1917; ccurrey@iastate.edu.
Visit our corporate website at https://hortamericas.com
 |
|
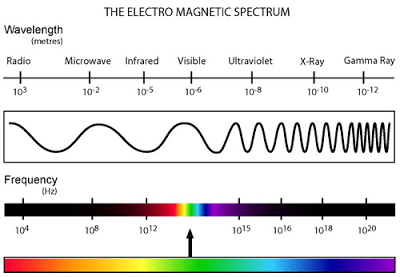
Figure 1. The electromagnetic spectrum. The visible spectrum encompasses approximately 380 nm to 780 nm. Photosynthetically active radiation or PAR is 400 nm to 700 nm.
|
 |
|
Figure 2. Image depicting photons of light falling on one square meter.
|
 |
|
Figure 3. Map of outdoor DLI by month throughout the United States.
Visit our corporate website at https://hortamericas.com.
|